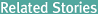|
|
Deputy Prime Minister and Minister of Strategy and Finance meets with Samsung Electronics Vice Chairman Lee Jae-yong at a semiconductor plant in Pyeongtaek, Gyeonggi Province, on Aug. 6. (joint photo pool)
|
Samsung asks government to lower prices of pharmaceuticals
Samsung Electronics Vice Chairman Lee Jae-yong recommended that the South Korean government increase the price of new and innovative drugs under the national health insurance system – a recommendation that unexpectedly coincides with a longtime desire of American multinational pharmaceutical companies, an objective they have constantly sought to achieve through the South Korea-US Free Trade Agreement and other means. Samsung’s interests happen to overlap with those of the American pharmaceutical industry: Lee wants the pricing of original drugs to be left to the mechanism of market competition, which is likely to provoke controversy since it echoes the American argument that drug pricing decisions should be entrusted to an independent private board instead of the public health authorities. Industry sources told The Hankyoreh on Aug. 7 that Koh Han-seung, president of Samsung Bioepis, joined Samsung Electronics Vice Chairman Lee Jae-yong for a meeting with Minister of Strategy and Finance and Deputy Prime Minister Kim Dong-yeon on Aug. 6. During this meeting, sources said, Koh asked the government to lift a regulation that requires the price of original drugs to be lowered when Samsung Bioepis releases biosimilar copies. Koh wants the system to be amended so that decisions about the price of the original drugs (which are generally produced and sold by multinational pharmaceutical firms) are determined by autonomous competition and bidding in the market, which would effectively allow drug prices to go up. The logic is that this would give Samsung’s biosimilars a boost in competing with original drugs in the domestic pharmaceutical market. “Bizarrely enough, Lee’s request basically reiterates the longtime American desire to raise the price of original drugs,” said Woo Seok-gyun, the policy committee chair for the Korean Federation of Medical Activist Groups for Health Rights. Under the health insurance drug pricing system, the price of biosimilar drugs is generally capped at 70 percent of the price of the original medicine before the patent expired, but since Oct. 2016, that cap has been raised to 80 percent for biosimilar drugs produced by “innovative pharmaceutical firms.” Since the authorities have already raised the price of biosimilar drugs once, Samsung would be hard-pressed to ask for another price hike. That’s apparently why it’s asking for an increase in the price of the original drugs, something that multinational pharmaceutical firms have long been requesting. There are currently more than 20,000 medical products that are eligible for coverage under the national health insurance scheme, and most of the more than 5,000 original drugs included here are sold by multinational pharmaceutical firms. Samsung’s request contains a two-pronged argument: first, guaranteeing the price of Samsung Bioepis’s biosimilar products by increasing the cap, and second, leaving drug pricing decisions to the market mechanisms of free competition and bidding. The proposed overhaul of the drug pricing system is consistent with a request that American multinational pharmaceutical companies have repeatedly made through the KORUS FTA for drug prices to be determined through market competition and for an independent civilian board to be established to decide drug prices. The American preferences were incorporated into Chapter 5 of the KORUS FTA, which guarantees that drug prices will be based on competitive market prices and states that a private review board that is independent of South Korea’s public health authorities is to be established and maintained to recommend and decide drug prices. The US claims that South Korea has not implemented this part of the agreement and has been pressuring Seoul to do so. Since the time of the negotiations for the KORUS FTA in 2006, American multinational pharmaceutical firms have complained that South Korea’s health insurance prices for innovative new drugs are set too low and have called for the correction of artificial prices for various intellectual property rights, including new medicine. Since the position of these pharmaceutical firms is that price decisions for original drugs should be left to the market, Lee’s demand strikes some as being oddly reminiscent of the pressure exerted by the US trade representative. By Cho Kye-wan, staff reporter Please direct comments or questions to [english@hani.co.kr]