 |
|
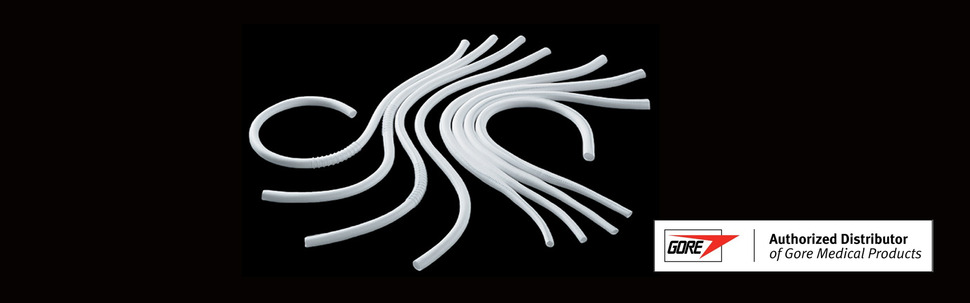
A vascular graft from Gore
|
Company demands exemption from GMP standards and that its products are sold at US prices
The US headquarters of Gore, a manufacturer of therapeutic materials, has placed the following conditions on resupplying vascular grafts to South Korea: exemption from regulatory documents and certification under the Good Manufacturing Practice (GMP) standards for medical devices and a guarantee that its products can be sold at the list price used in the US. Vascular grafts are artificial blood vessels that are necessary for pediatric heart operations such as the Fontan procedure. Gore made the request in an official letter sent to Korea’s Ministry of Food and Drug Safety on Mar. 8. After a number of Fontan procedures were postponed recently because Korean hospitals had exhausted their supply of vascular grafts, Gore said it was considering resuming its supply of the material to the South Korean market. This was the first official document in which Gore explained why it had withdrawn from the Korean market in October 2017 and stopped supplying vascular grafts. On Mar. 13, the office of Democratic Party lawmaker Kim Sang-hui provided the Hankyoreh with documents related to vascular grafts that had been exchanged by Gore, the Ministry of Food and Drug Safety and the Ministry of Health and Welfare. In its response to the Korean government’s request for help with the supply of vascular grafts, Gore said it was “very concerned about the humanitarian interest in our withdrawal from the Korean market.” At the same time, the company made clear to the Korean government what the conditions were for resuming the supply. “If Seoul agrees to waive the GMP review and regulatory documents and to sell the products at the US list price, we’ll give more ground and consider resuming the supply of vascular grafts,” the company said. The list price in the US of the vascular grafts that Korean hospitals are currently running out of is about 820,000 won (US$724.48), which is double the Korean sales price of 460,000 won (US$406.60). About 50 of these products are used in Korea every year. When the Korean government lowered the maximum price for therapeutic materials across the board, the price of this product was reduced by about 19%. Before it will resume its supply, Gore said, customs fees and shipping costs must also be included in the sales price. Sales prices can be raised on ”rare and essential therapeutic materials” Vascular grafts are categorized as a “rare and essential therapeutic material,” which makes it possible for their sales price to be raised. In September 2018, after Gore withdrew from the market, the Ministry of Health and Welfare changed the standards for calculating the maximum price of rare and essential therapeutic materials in its rules for paying medical costs as part of the country’s national health insurance. This change makes it possible for the health insurance price of Gore’s vascular grafts to be set in consideration of the retail price in the country of import and the manufacturing and import costs. Some aspects of the rationale behind Gore’s withdrawal from the Korean market in 2017 were also revealed in these documents. According to documents sent to Gore by the Ministry of Food and Drug Safety met with Gore representatives about the domestic supply of vascular grafts in June 2017, the Ministry of Health and Welfare attempted to dissuade Gore from leaving the market by promising to consider adjusting health insurance payouts. But Gore refused to make additional investments in the country, citing the cost of renewing its GMP certification, which would be expiring on Aug. 22, 2019. The Ministry of Food and Drug Safety interpreted this as meaning that Gore’s headquarters in the US is willing to supply the products even after GMP certification expires, but that it was reluctant to pay for GMP certification given the small number of patients in the country, which would make it hard to turn a profit. Unclear why Gore withdrew from Korea while continuing to supply to Europe Gore’s request for an exemption from GMP certification and regulatory documents in its letter to the Ministry of Food and Drug Safety on Mar. 8 also appears to be related to the reason it withdrew from Korea in 2017. However, it remains unclear why Gore only left Korea while still supplying products to Europe, which has higher costs and tougher regulations, including the frequency of recertification. The Ministry of Food and Drug Safety explained that it costs 2.5 million won (US$2,207) to renew GMP certification in South Korea every three years, while certification in Europe costs 15 million won (US$13,241) and takes place every year. During the Ministry of Food and Drug Safety’s work briefing at the National Assembly’s Health and Welfare Committee on Mar. 13, lawmakers from both the ruling and opposition parties slammed the ministry for its failure to be more proactive about resolving this issue. “The Ministry appears to have been twiddling its thumbs for the two years following Gore’s withdrawal. While it’s fortunate that Gore has agreed to supply 20 vascular grafts for now, we need to solve the problem of resuming and sustaining the supply of these products and taking thorough measures to ensure that nothing of this sort happens again,” Rep. Kim Sang-hui said. Vice Minister of Food and Drug Safety Choi Sung-rak noted that the Ministry had “taken some measures, such as restoring the import permit and revising related laws” in regard to the product licenses that Gore had voluntarily dropped, but expressed his “remorse” for the occurrence of this incident. “We’re planning to have a video chat with Gore this week, and we’ll swiftly implement fundamental improvements to ensure that similar incidents do not occur,” Choi said. By Hwang Ye-rang and Kim Yang-joong, staff reporters Please direct comments or questions to [english@hani.co.kr]