Posted on : Mar.14,2019 16:48 KST
Modified on : Mar.14,2019 17:06 KST
 |
|
Minister of Food and Drug Safety Lee Eui-kyung answers questions from the National Assembly’s Health and Welfare Committee regarding Gore’s withdrawal from the South Korean market on Mar. 13. (Kim Gyoung-ho, staff photographer)
|
Regulations cited as reason for pulling out by company are stricter in other countries
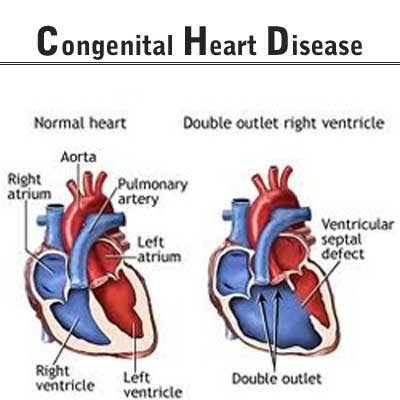
Children with congenital heart defects are facing a delay in operations as stockpiles of Gore products have recently begun to run out following the US company’s move in October 2017 to stop supplying vascular grafts (artificial blood vessels) and other medical items needed for heart surgery to the South Korean market. A path for some patients to undergo surgery in the near future was cleared on Mar. 11 when Gore announced it would be supplying 20 grafts to meet urgent needs – but it remains unclear whether the supplies will continue going ahead. The circumstances behind Gore’s April 2017 decision not to provide any more vascular grafts in South Korea warrant some examination, if only to ensure no future situations along the same lines.
According to an email from Gore to the South Korean Ministry of Food and Drug Safety (MFDS) acquired on Mar. 13 by the Hankyoreh, the company demanded an increase in vascular graft prices and exemptions on Good Manufacturing Process (GMP) examinations during its discussions with the MFDS on resuming supplies of the grafts. This issue is believed to be what led Gore to withdraw from South Korea in 2017. Some maintain that reductions on treatment material costs and the strictness of the GMP examinations left Gore with no choice but to pull out – but several questions also remain.
Was the SK government justified in lowering treatment material costs prior to withdrawal?
 |
|
congenital heart disease
|
In 2016, the Ministry of Health and Welfare (MOHW) decided to reduce health insurance prices for vascular graft products by around 19%. For medical treatment materials, domestic health insurance prices are decided by an expert assessment committee based on factors including the manufacturer’s production cost and sales data. According to details obtained by the Korean Society for Thoracic and Cardiovascular Surgery (KTCVS), the cost of a vascular graft in South Korea at the time was around 460,000 won (US$406.10) – a major difference from the equivalent of around 820,000 won (US$723.86) in the US and 1.47 million won (US$1,298) in China. The cost fell along the same lines as Taiwan, which operates a similar health insurance system to South Korea’s. Some analysts have commented that differing medical systems make direct comparison of treatment component costs difficult; others have argued that prices should be lowered on products developed a long time ago, as there is no longer a need to reflect R&D costs.
An MOHW examination of the prices of vascular grafts actually sold in South Korea’s healthcare market in 2014 showed the items to be trading for over 20% lower than the price set by the National Health Insurance Service (NHIS).
“Like medications, treatment materials also fall under a system where prices are re-determined on the market to reflect actual transaction prices,” explained Lee Joong-gyu, head of the MOHW’s insurance payment division.
“The costs of other treatment materials also decreased as a reflection of real transaction prices,” Lee noted.
Procedures were also put in place for manufacturers to raise issues concerning the reduced prices with an appeal organization independent from NHIS. Gore did submit an objection over the price reduction to the organization, but it was not accepted.
“When the prices of medications and treatment materials are set too high, it becomes difficult for patients to visit the hospital due to the financial burden,” explained Kang Ju-seong, co-director of the group Health Right Network.
“It also has a negative effect on NIHS finances, which means they have to raise premiums. Every country has procedures to allow reductions in the prices of medications and treatment materials,” Kang said.
Procedures established in September 2018 to accept US prices
As the reduced market transactions prices for treatment materials resulted in problems with an inability to supply rare and essential components such as vascular grafts, MOHW moved to improve the system. In September of last year, it announced calculation standards aimed at ensuring a suitable price for such components.
“It was amended to allow foreign manufacturers to receive the same sales price in South Korea that they would in the corresponding country,” said Lee Joong-gyu.
“Gore could receive the same US sales price for vascular grafts in South Korea if they requested that,” Lee added. But Gore has yet to go through procedures to resume supplies.
Manufacturing process examinations tougher than elsewhere?
MFDS permission is required before a treatment material can be sold or used in South Korea. Once approval has been granted, the ministry visits manufacturers every three years to conduct GMP examinations to ensure continued quality. For companies to pass these examinations, they are required to improve their facilities. Observers in the medical community also suggested the risk of business secrets leaking during the examinations may have been a major burden for Gore.
“Other places like Canada and countries in Europe also have manufacturing process examinations,” said an MFDS official.
“You could argue that it’s even more stringent there than in South Korea, since they do the examinations every year,” the official added.
The ministry also maintains that the examination costs in South Korea are far lower than in Europe. Since Gore received a three-year approval in 2016, it was not in a position where it needed to make an immediate decision to withdraw, the ministry suggested.
Did MFDS approval need to be revoked?
The revocation of Gore’s vascular graft approval when it pulled out of South Korea resulted in difficulties reintroducing the items. In February 2018, KTCVS requested that MFDS restore the approval. The ministry demanded that the company go through official procedures to newly register the grafts as treatment materials; Gore declined to submit the necessary documents, and the approval was not granted. Instead, MFDS has maintained that items with no available alternatives in South Korea can be imported and used if patients receive “written confirmation of medical equipment for test purposes” for individual use. Due to the lack of importing businesses, patients were still unable to acquire the vascular grafts, prompting MFDS to grant importation company approval in February to allow supplies of the grafts.
But because of restrictions stating that Gore products sold in a given country may only be used in that country, vascular grafts supplied to Taiwan and elsewhere cannot be imported into South Korea.
Woo Seok-gyun, co-representative of the group Association of Physicians for Humanism, said that Gore “should be held responsible for declining to supply treatment materials to patients who need surgery right now because of cost and process review reasons, while making it so they cannot be obtained through other means.”
By Kim Yang-joong and Hwang Ye-rang, staff reporters
Please direct comments or questions to [english@hani.co.kr]